Our Science
Neuronal Hyperactivity in various brain regions is associated with anxiety, depression, and other neurocognitive disorders and psychiatric conditions.

Article by Dean Radin, Ph.D., Chairman and Co-founder
in Springer Nature | Resarch Communities
Toward a new class of genetic therapeutics for mental health disorders
Read Article
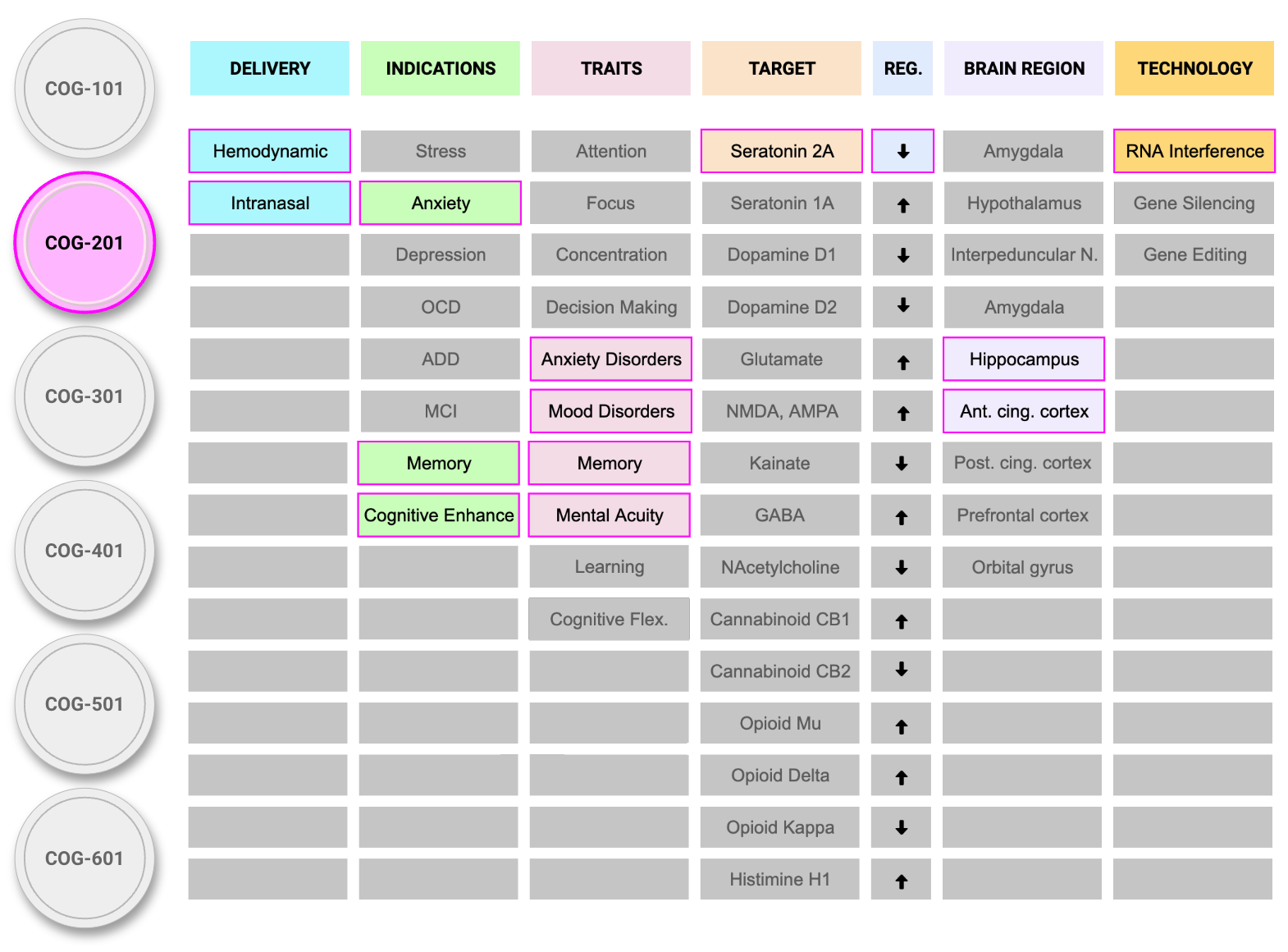
RNA Therapeutics, Engineered for Precision Neuromodulation
At Cognigenics, we are building the next generation of psychiatric treatments by integrating molecular genetics, neuroscience, and targeted delivery technologies.
Our unique AcuGen platform centers on short interfering RNA (siRNA)—a highly selective therapeutic modality that allows us to modulate gene expression with unprecedented spatial and temporal precision. Delivered via lipid conjugates, our therapeutics reach the brain noninvasively through intranasal administration, bypassing the blood-brain barrier.
Target: 5-HT2a Receptor
Decades of pharmacological and imaging research have implicated 5-HT2a receptor overactivation in limbic and cortical circuits as a key mechanism underlying hallucinations and psychosis, particularly in Parkinson's Disease Psychosis. The clinical efficacy of selective 5-HT2a antagonists and evidence of upregulated receptor density in affected brain regions support this.
Our approach is a fundamental change. Instead of the outside-in approach of traditional small-molecule drugs, we're developing an inside-out approach, modulating only specific neuronal receptors in the brain.
COG-301 silences 5-HT2a gene expression at the mRNA level using siRNA, enabling receptor-specific downregulation without polypharmacology.
Cognigenics Therapeutic Strategy – An Evolution
Our scientific development has progressed through three strategic phases:
- CRISPR Knockout
Used to establish causality between specific gene targets and behavioral phenotypes. - shRNA Gene Silencing
Provided long-duration suppression and validated therapeutic impact in preclinical models. - siRNA + Lipid Conjugates Delivery (Current Focus)
Enables tunable, reversible modulation. Ideal for dynamic conditions like PDP with co-occurring anxiety, allowing dose control and flexible treatment schedules.
THE COGNIGENICS PLATFORM
Our intranasal delivery platform offers a novel way to provide permanent (via DNA) or long-lasting (via RNA) treatments for mental health disorders associated with hyperactive neurons. The platform can accommodate a wide range of genetic therapeutics, enabling precise treatments that can be customized for individual needs.
Precision Delivery via Intranasal Lipid Conjugates
Our patent-pending lipid conjugates formulation will enable nose-to-brain targeting—a direct path to the limbic and cortical regions implicated in psychiatric illness. Intranasally delivered, this formulation is designed to:
- Avoid systemic exposure
- Enable weekly dosing
- Require no implanted hardware or infusion devices
- Allow at-home patient administration and cold storage in a standard refrigerator
This approach balances efficacy, safety, and scalability in a way conventional psychiatric treatments cannot.
Advancing a New Class of CNS Therapeutics
We believe Cognigenics is defining a new category of neurotherapeutics:
Precise. Reversible. RNA-based.
COG-301, our lead candidate under development, embodies this innovation. It's a first-in-class RNAi therapy for anxiety and perceptual symptoms in PDP—and it lays the foundation for broader expansion across psychiatric and neurodegenerative indications.
